Understanding the Micronization of APIs
In the synthesis process, APIs for oral solid dosage are often obtained by crystallization. However, in many cases, the particle size distribution of the crystallized APIs often does not meet the needs of the drug efficacy. Therefore, the APIs need to be further milled to control the particle size within the target range.
The micronization of active pharmaceutical ingredients is a process used to reduce the particle size of the drug substance to the micron level, typically less than 20 microns in diameter. For some inhaled drugs, the particle size is usually less than 7 microns that can be delivered deeply into the lungs.
the Purpose of Micronization of APIs
The micronization technique is commonly employed in the pharmaceutical industry for several reasons.
Enhanced Solubility
Smaller particles have a greater surface area relative to their volume, which can improve the solubility and dissolution rate of poorly soluble drugs. This is crucial for drugs that need to be rapidly absorbed in the digestive tract.
Improved Bioavailability
By increasing the dissolution rate, micronization can enhance the bioavailability of the drug, meaning a higher percentage of the drug reaches the bloodstream and can exert its therapeutic effect.
Consistent Dosage
Micronized APIs can lead to more uniform mixing with excipients, facilitating consistent dosage in each tablet or capsule.
Better Control Over Drug Release
Smaller particle sizes can help in achieving desired release profiles for controlled or sustained-release formulations.
The micronization of APIs can be divided into dry grinding and wet grinding based on the dispersion medium. Dry grinding refers to comminution conducted in a gas environment. Wet grinding involves the comminution of raw materials in a liquid medium. APIs are generally ground in dry methods. The most popular equipment for the micronization of APIs is a jet mill which grinds drug particles into finer particles through high-speed air flow.
Example of Micronization
Rifampin is an antibiotic that is primarily used to treat bacterial infections, most notably tuberculosis and leprosy. Rifampin is available in various forms including capsules, oral suspensions, and intravenous formulations.
Equipment used: Spiral jet mill
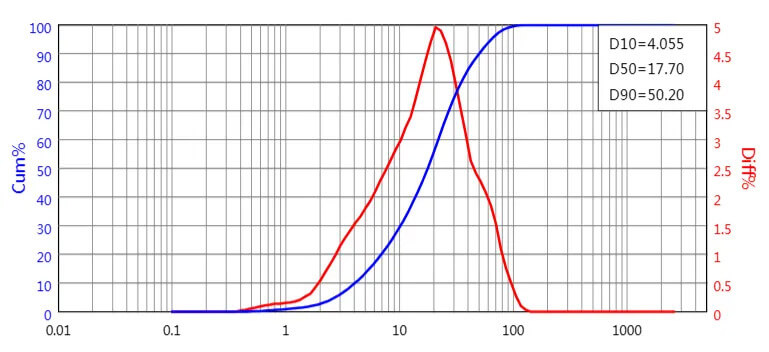
Particle size before grinding: D50=17.70µm, D90=50.20µm
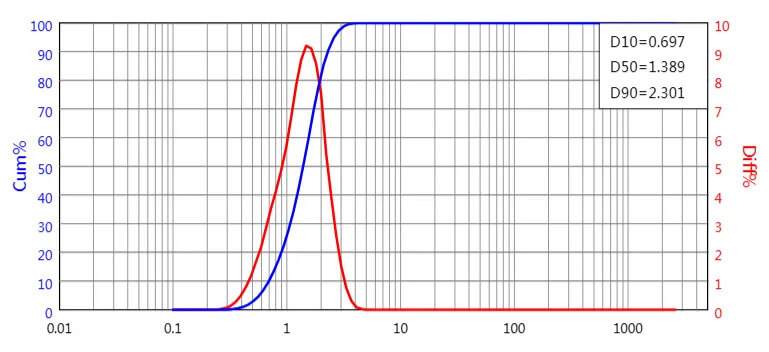
Particle size after grinding: D50=1.389µm, D90=2.301µm
PSD before micronization
PSD after micronization
Temperature Control
Temperature control is crucial to ensure the quality and stability of pharmaceuticals. Many APIs are heat-sensitive, and maintaining specific temperature ranges throughout the production process is essential to prevent degradation and ensure product efficacy.
Additionally, equipment surfaces that come into direct contact with the APIs must be carefully maintained to prevent any temperature-induced degradation. During the micronization process, the adiabatic expansion of compressed gas leads to the Joule-Thomson cooling effect, which helps maintain the temperature of the entire grinding chamber between from 5°C to 20°C.
Inert Gas Protection
Inert gas protection refers to the process of using Nitrogen or Argon to isolate the air in the production process such as grinding, mixing and packaging of active pharmaceutical ingredients. The purpose of using Inert gases are as follows:
- It is necessary to control air humidity to maintain the quality of the APIs.
- It helps prevent oxidation and degradation of the APIs or other reactive substances.
- For unstable APIs, it can effectively improve production safety.
Containment
Highly Potent APIs (HPAPIs) are a class of drug substances that remain potent at low doses, producing significant pharmacological effects. The risk from highly potent APIs is based on an occupational exposure limit (OEL) or an occupational exposure band (OEB).
The containment systems are critical for ensuring the safety of personnel and the environment, as well as maintaining the integrity of the product. The design of containment system depends on various factors, including the potency and toxicity of the HPAPI and the specific processes involved.