The Ultimate Guide to Micronization
Why is Micronization Important?
Micronization is important for various reasons.
Increased Surface Area
By reducing the particle size to the micron level, the surface area of the substance is significantly increased. This can enhance the dissolution rate, bioavailability, and overall effectiveness of pharmaceuticals and other products.
Improved Mechanical Properties
Ultrafine powders can exhibit unique mechanical properties such as increased strength, ductility, and hardness, which can be beneficial in advanced material applications.
Enhanced Optical Properties
The small size of ultrafine particles can lead to interesting optical effects, such as improved color saturation, increased light absorption, and unique light scattering properties.
Greater Chemical Reactivity
Ultrafine powders often have higher chemical reactivity due to their increased surface area, making them useful in catalysis, chemical reactions, and material synthesis.
Overall, micronization plays a vital role in enhancing the performance, quality, and usability of a wide range of products across various industries.
Methods of Micronization
Micronization methods can be divided into two main categories: traditional techniques and modern techniques.
Traditional Techniques
Dry grinding and wet grinding are two common methods used for particle size reduction. Dry grinding involves the process of reducing particles to smaller sizes without the presence of any liquid or solvent. Wet grinding is the process of reducing particles to smaller sizes using a liquid or solvent to aid in the breakdown of the material.
Jet Mill
A jet mill, also known as micronizer, is a grinding equipment used in super fine particle size reduction. In a jet mill, particles are introduced into a high-speed stream of gas inside a grinding chamber. The kinetic energy in the stream of gas causes interparticle collision, and as the high-velocity particles hit against each other, they break apart, creating finer particles.
Advantages of Jet mills
- Fine particle size and uniform distribution
- Suitable for heat-sensitive material at a low temperature
- High product purity due to minimal or no mechanical wear
Disadvantages of Jet mills
- Require compressed air as an energy source
- Not suitable for high ductile substance
Air Classifier Mill
An air classifier mill combines a mechanical mill with an internal air classifier in a single machine. The material is fed into the mill and impacted by hammers or pins rotating at high speeds. After the grinding process, the air classifier inside the mill separates the particles based on their size. This classification process allows for the controlled size distribution of the final product.
Advantages of Air classifier mills
- Combines Grinding and Classification
- Uniform Particle Size Distribution
- Relatively low energy consumption
Disadvantages of Air classifier mills
- Significant initial investment cost
- Heat generated during the grinding process
- Require regular maintenance and replacement of parts
Ball Mill
A cylindrical shell rotates around its horizontal axis. As the ball mill rotates, the grinding media (balls) inside it impact and grind the material into a fine powder. The balls impact the material, causing it to break into smaller particles through impact and attrition forces. The rotation speed of the ball mill can be controlled to achieve optimal grinding performance.
Advantages of Ball mills
- Continuous operation for large-scale production
- Easy to operate and maintain thanks to a simple design
Disadvantages of Ball mills
- Require long grinding time
- Potential for contamination due to wear of the grinding media
- Significant noise and vibration
Bead Mill
Bead mills are primarily used for wet grinding processes where the material being processed is in a liquid or slurry form. The grinding media and the material are agitated in the mill, leading to particle size reduction and dispersion. Bead mills are effective in reducing the size of particles to achieve a finer and more uniform particle size distribution. Bead mills are commonly used for dispersing pigments, dyes, and other additives in liquid formulations.
Advantages of bead mills
- Suitable for ultra-fine processing
- High grinding uniformity
Disadvantages of bead mills
- High energy consumption during operation
- Maintenance challenge due to complex internal structure
- Challenges in bead size and material selection
Colloid mill
A colloid mill consists of a rotor and a stator that work together to process the materials. The high-speed rotation creates intense shear forces that reduce the size of solid particles in a liquid. Colloid mills are effective in reducing the particle size of solids and achieving a fine dispersion in liquids. The higher the shear, the finer the resulting particle size. They can process materials such as solids suspended in a liquid, semi-solids, and liquids containing solid particles.
Advantages of colloid mills
- Suitable for highly viscous products
- Adjustable gap settings between rotor/stator for particle size control
- CIP and SIP available
Disadvantages of colloid mills
- Not suitable for large-scale production
- Prone to wear and tear
High Pressure Homogenizer
A high-pressure homogenizer consists of a positive displacement pump that generates high pressure to force the material through a narrow orifice or valve. As the material passes through the narrow gap, the intense pressure and velocity create shearing forces that further break down particles into smaller sizes. Additionally, the sudden drop in pressure after passing through the orifice can induce cavitation, leading to additional particle disruption.
Advantages of High-pressure homogenizers
- High uniformity and stability of final products
- Various applications such as emulsification, dispersion, and cell disruption
Disadvantages of High-pressure homogenizers
- Significant amount of energy
- Not suitable for high viscosity products
- High requirements for mechanical sealing
Comparison of Traditional Micronization
| Method | Dry / Wet | End Fineness | Application |
| Jet Mill | Dry Grinding | 1 µm – 50 µm | Used for micronizing of solids in pharmaceuticals, chemicals, food, cosmetics, minerals |
| Air Classifier Mill | Dry Grinding | 10 µm – 400 µm | Mass continuous production, suitable for mineral and chemical industry |
| Ball Mill | Dry Grinding | 0.1 µm- 30 µm | Widely used for grinding material such as cement, coal, limestone and pigment |
| Bead Mill | Wet Grinding | 50 nm – 10 µm | Typically used in the production of coatings, inks and various chemicals |
| Colloid Mill | Wet Grinding | 2 µm – 40 µm | Fine grinding and emulsification in food, cosmetics and chemical industry |
| High Pressure Homogenizer | Wet Grinding | 1 µm – 5 µm | Homogenization in the production of dairy products, cosmetics, pharmaceuticals |
Modern Techniques
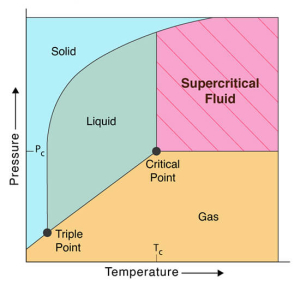
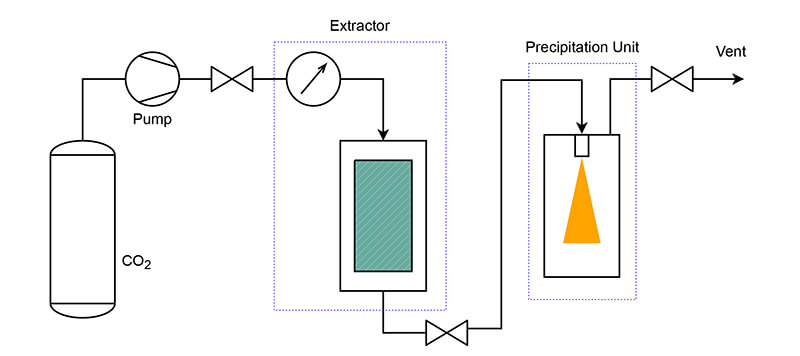
Supercritical fluid technology is an advanced micronization technique that leverages the unique properties of supercritical fluids to achieve fine particle size reduction. A supercritical fluid is a substance at a temperature and pressure above its critical point, where it exhibits properties of both liquids and gases. Carbon dioxide is the common supercritical medium due to its low critical temperature and pressure, non-toxicity, non-flammability.
Rapid Expansion of Supercritical Solution (RESS)
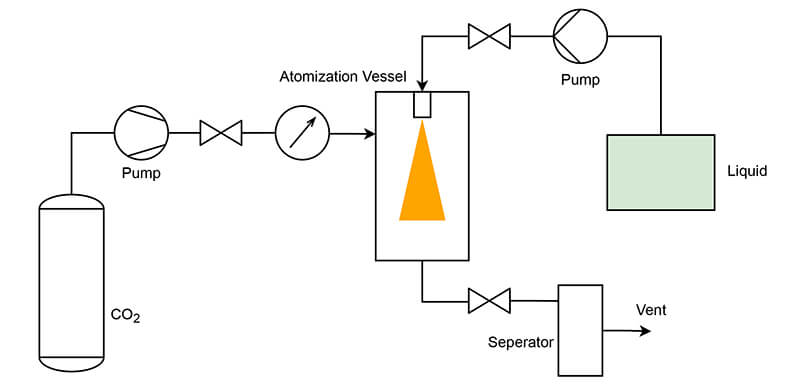
In the RESS process, organic material is dissolved in scCO2 and then rapidly expanded through an expansion nozzle, resulting in a sudden pressure drop to atmospheric levels. This causes the dissolved substance to precipitate as fine particles. The RESS technique is effective for producing uniform and pure micronized particles, especially for thermally sensitive materials.
Supercritical Anti-Solvent (SAS)
SAS mainly micronizes substances that are insoluble in supercritical fluids. When a supercritical fluid is mixed with a solution containing a dissolved substance, the supercritical fluid reduces the solubility of the substance, causing it to precipitate as fine particles.
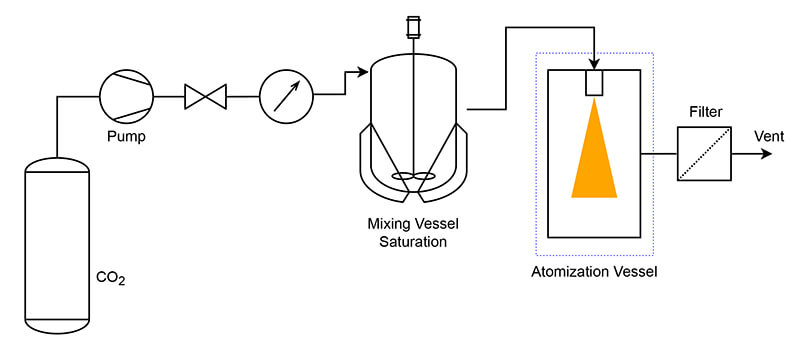
Particles from Gas Saturated Solution (PGSS)
The substance is first dissolved in a liquid solvent. The solution is then brought into contact with scCO2 at high pressure. The saturated solution is then rapidly expanded through a nozzle into a low-pressure chamber. Due to the drop in temperature and solubility, the solute precipitates out of the solution, forming fine particles.
Comparison of Modern Techniques
| Item | RESS | SAS | PGSS |
| Role of SCF | Solvent | Anti-Solvent | Solute |
| Dring force | Pressure | Solubility | Temperature |
| Working Pressure Dependence | SCF | SCF | Morphology |
| Working Temperature Dependence | SCF | SCF | Highest |
| Particle Size | Micro & Nano | Micro & Nano | Micro & Nano |
Future Trends
We are faced with more challenges to meet the more demanding requirements. Future trends in micronization are expected to focus on innovation, sustainability, efficiency, and customization.
Nanotechnology Integration
With the increasing demand for nanoscale materials, micronization processes will integrate nanotechnology principles to produce nanoparticles with specific properties.
Online control of product fineness
By implementing the online monitoring and control system, we make sure the production of high-quality micronized products with the desired fineness characteristics.
Product purity improvement
Reducing product contamination is a constant issue. Improving product purity is essential to ensure the quality and meet regulatory requirements.
Reduction of energy consumption
Green micronization processes will offering advantages such as reducing energy consumption and minimizing waste generation.